Below are the One-Plate® Plating Rates for Mid-Phos, High-Phos, and Low-Phos.
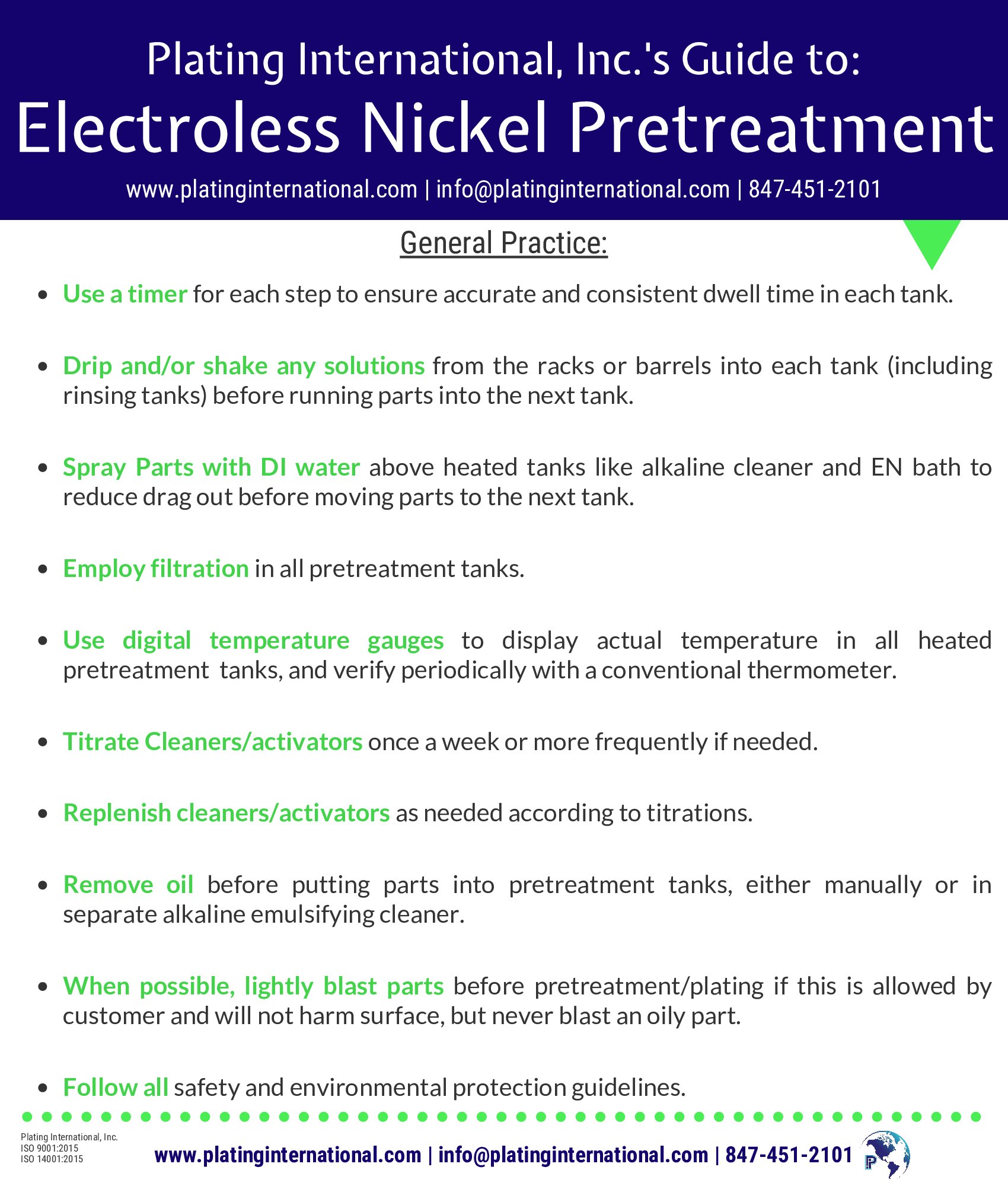
Plating is only part of the process. In order to ensure that your parts are plated to the best quality, follow Plating International, Inc.’s Guide to Pretreatment for Electroless Nickel!
Did you know companies are finding the benefits of One-Plate® are even greater as one component is easier to order, ship, and stock than three components.
Shops are finding this very convenient especially now that supply chains are strained by transport issues.
Shops with short labor or split shifts are reporting how One-Plate® is easier to operate with less workers and lab analysis.
And for those shops where their business level is lower due to the pandemic, they are appreciating the savings in time, energy, labor, and costs.
One-Plate® is now in 15 countries on 5 continents.
Plating International has increased our manufacturing capacity to meet the growing demand.
Single component One-Plate® EN versions are now available in low, mid, high phos and composites with Teflon, diamond, silicon carbide and boron nitride.
Just as the cold weather is coming, Plating International has verified the freeze resistance of the most popular One-Plate® 1001Q solution. Attached please see a photo with a typical "A" solution on the left and One-Plate® 1001Q on the right. After two weeks in a 5 degree F (-15 C) freezer, the "A" solution is frozen solid. The One-Plate® solution was just very cold. We then tested the One-Plate® solution and it plated as well as it always does, so there was no negative affect from the cold storage.
New technical articles and case studies are in the works..
Also, in this Covid-19 time when travel is limited, we have been having frequent video conference meetings to support plating shops and prospective customers to answer any questions they have, please don’t hesitate to reach out to set up any calls like this.
Plating International Inc.’ s new video discusses why Bob the chrome plater is ready to make the switch to a Flex Kraft switch mode rectifier from his traditional SCR rectifier. Click the video below to learn more!
Traditionally, most chrome baths use lead anodes. When we think of lead anodes, we usually just think about the lead part, but in fact the lead anodes that are used are never made of pure lead. Chromic acid in the bath eats away at pure lead, especially when there is no current. Pure lead is also quite soft, does not hold its shape very well and sags under its own weight. This is why lead is alloyed with other metals in order to give it specific properties. Various amounts of antimony, tin and silver are added to the lead depending upon the application.
Antimony: Provides hardness, rigidity and resistance to curling or sagging and is used whenever strength is required. High antimony contents, however, tend to produce excessive surface scale and a less than optimum trivalent control. Antimony has a density of 0.24 lbs. per cubic inch and a melting temperature of 1170 degrees F.
Tin: Provides improved corrosion resistance and conductivity, reduces surface scaling and improves trivalent control. Used primarily in high fluoride baths. Tin has a density of 0.26 lbs. per cubic inch and a melting temperature of 450 degrees F.
Silver: A small amount of silver (0.5 - 1%) greatly extends the corrosion resistance and increases the conductivity. Due to the additional cost, this is used only where an extended anode life is required such as in very high fluoride baths.
C. P. Grade Lead: CP grade lead (99.9 % chemically pure) is the basic material that is used to make the various alloys. CP lead has a density of 0.41 lbs. per cubic inch and a melting temperature of 620 degrees F.
Lead Alloys: The anode materials are purchased from a smelter already alloyed per specification. These materials are available in ingots, cast mats, rolled sheet & bars, extruded pipe and extruded rods or wire in various sizes. Extruded and rolled forms are much denser than cast materials are and will therefore hold up much longer and are better suited for large anodes or ones that need to last for long periods of time.
It is good practice to standardize alloys and use only one type per bath. If several alloys are used then the each type should be marked so they are not accidentally mixed. Lead alloys should never be obtained from a scrap dealer as the quality is unknown. Most lead alloys used for chrome plating have a density of around 0.40 lbs. per cubic inch and a melting point of 580 – 600 degrees F.
6% antimony - 94% lead: This is a very common alloy that is used for a majority of chrome plating anodes. The antimony provides both hardness and rigidity and is particularly well suited for large or heavy anodes. The surface film from this alloy provides reasonable control of the trivalent, but the scaling is heavier than if tin were present.
7% tin – 93% lead: Used in all type baths including high fluoride solutions. This alloy is softer than 6/94 is and may sag if too heavy or too large. This alloy has an improved peroxide surface film for better trivalent control and reduced scaling.
2% tin – 4% antimony – 94% lead: This alloy provides a combination of improved rigidity and corrosion resistance. It has a better surface film than 6/94 does, but not quite as good as the 7/93 alloy is. It is used where a combination of optimum strength, resistance to distortion and surface film is needed.
0.5% silver – 4% tin – 2% antimony – 93.5% lead: The addition of a small amount of silver greatly improves the surface film and increases the corrosion resistance. The silver content is typically 0.5%, although it can be as high as 1% for even greater benefit. This alloy typically lasts 2-3 times longer than the others do. Obviously, the cost of the silver must be weighed against the value of the additional benefits obtained. This alloy is used primarily in very high fluoride baths.
Properly passivated stainless steel will not plate unless contacted by a plating part or the bath is not properly maintained. They must be passivated from time to time according to the procedures established (by STI) for safety and effective passivation without contaminating the solutions or rinse tanks. Stainless steel is composed of iron (Fe); nickel (Ni); chromium (Cr) and several other minor components. Stainless steel is not resistant to chemical or physical attack. The corrosion resistance of stainless steel depends on the formation of a ‘passive surface film’ composed of nickel and chromium oxides (Cr2O3 & Ni0).
Procedure:
1. Pump bath out into the corresponding drums, tote, or tank.
2. Spray rinse the tank & plumbing, then drain any leftover bath into corresponding drums or tank.
3. Pump in 40% Nitric for Passivation from the tote into empty tank.
4. Once tank is filled with “40% Nitric for Passivation” do the following 3 things.
A. Turn on all pumps to tank.
B. Set temperature of the “40% Nitric for Passivation” to 90o F.
C. After you have completed Set 1 and 2 set timer for 3 hours.
5. After 3 hours are up, unplug all pumps in the tank and turn off steam,
6. Pump “40% Nitric or Passivation” into the tote.
7. Drain any leftover Nitric into a bucket through the valve behind the tank and pour back into tote.
8. Spray rinse the tank and plumbing, then drain straight to the trench.
9. Fill empty tank with city water.
10. After the tank is filled with city water do the following 3 things.
A. Turn on all pumps to tank
B. Turn on air agitation
C. Add 2000 mls of NH4OH to neutralize water (per 100 gallons of water)
11. Let mix for 10 mins.
12. Pump water back into the tote unless instructed otherwise.
13. Spray rinse the tank and plumbing, then drain straight to the trench.
14. Take out spargers and clean in tank.
15. Thoroughly spray clean tank (Walls, Plumbing, Backflush, Etc.)
16. Once tank has been thoroughly cleaned reattach spargers back onto the reactor.
17. Spray rinse the entire tank with DI water one last time.
18. The tank has now been successfully passivated & cleaned and is now ready for a bath to be pumped in or made up new after the valve is closed.
One of the major decisions that plating shops have to deal with is “what kind of plating tank should I get for my plating line?” Of course, we wish the answer was as simple as the question, but the truth of the matter is it depends on a lot of different things. What are you plating? Plating chromic acid is a completely different process than zinc or cadmium plating. How much are you plating? 300 gallons or 3000 gallons? What method are you using to plate? There are so many questions to ask and factors to consider, we thought it would be nice to see it in the form of a pros/cons table:
As you will see in the diagram above there are many different things to consider when choosing a plating tank. Polypropylene tanks are great for smaller bath sizes and they are well-suited for the harsh conditions provided by plating shops. Polypropylene tanks are also very durable and they are great for use with acids and alkalies, which is why you can sometimes avoid buying a liner for your tank (unless you’re plating chromic acid, which you’ll definitely still need a liner for!) Polypropylene tanks are also much more flammable than steel tanks, which can be an issue if you are using electrical heaters to heat up your bath. While steel can hold up to well over +2000°F, polypropylene melts at around 320°F. Another factor is their size limit - because of polypropylene’s lower tensile strength and modulus, you will likely need supports to prevent bulging and breaks at the seams; but steel tanks have a lot more natural support because of their higher tensile strength. However, steel tanks are vulnerable to other conditions that the polypropylene tanks will almost never encounter: they can rust and they are susceptible to pinholes, which can be very dangerous if not immediately fixed.
At the end of the day, you’re really going to have to take the time and think about what solution works best for your business needs. That’s why you should ask the experts here at Plating International. With over 40+ of working experience, we can help you well above-and-beyond all your plating needs. Contact us today for a quote on plating tanks!